Virus Triggers a Key Molecule That Causes Neuronal Damage in HTLV-1-Associated Myelopathy ー New insights open the door to future therapeutic strategies targeting RGMa ー
- Headline
- Press Release
St. Marianna University School of Medicine
Graduate School of Frontier Sciences, The University of Tokyo
A research team led by Dr. Yoshihisa Yamano at St. Marianna University School of Medicine (Kawasaki, Japan) has uncovered a previously unknown mechanism by which human T-cell leukemia virus type 1 (HTLV-1) causes neuronal damage in patients with HTLV-1-associated myelopathy (HAM/TSP), a progressive and disabling neuroinflammatory disease.
The study demonstrates that HTLV-1-infected immune cells express a molecule called RGMa (Repulsive Guidance Molecule A), which blocks nerve regeneration and leads to neuronal injury. The findings suggest that blocking RGMa may offer a novel therapeutic approach for HAM/TSP.
These results were published in the journal JCI Insight in [Month] 2025.
Research Background
HTLV-1 is a retrovirus that infects CD4+ T cells and is known to cause adult T-cell leukemia/lymphoma (ATL) and HAM/TSP. In HAM/TSP, HTLV-1-infected immune cells infiltrate the spinal cord, triggering chronic inflammation and progressive neurological damage. Despite decades of research, the molecular mechanism by which these infected cells damage neurons have remained elusive.
Key Findings
- HTLV-1-infected T cells aberrantly express RGMa, a molecule that inhibits axon growth and neuronal repair.
- The expression of RGMa is induced by the HTLV-1 viral protein Tax, which works together with the host transcription factor Sp1.
- This mechanism is further supported by epigenetic deregulation in infected cells, specifically reduced H3K27me3 at the RGMa gene locus.
- In vitro experiments showed that blocking RGMa with a neutralizing antibody (unasnemab) significantly reduces the neuronal damage caused by HAM patient-derived immune cells.
"This study shows that HTLV-1-infected cells do not merely cause inflammation--they actively produce molecules that directly impair neurons," said Dr. Yamano. "Targeting RGMa could represent a new direction for treatment."
Implications for Treatment
The study identifies RGMa as a key mediator of neuronal injury in HAM, offering new hope for therapeutic development. Based on these findings, a Phase I clinical trial of the RGMa-neutralizing antibody MT-3921 is currently underway to evaluate its safety and potential to improve symptoms in patients with HAM.
Suggested Visuals
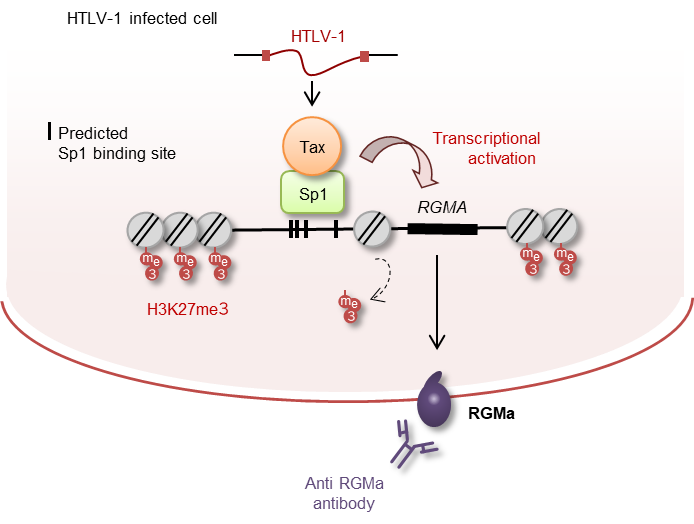
Figure 1. Diagram of the proposed mechanism: HTLV-1 Tax + Sp1 → RGMa expression → binding to neuronal receptor → inhibition of axon regeneration
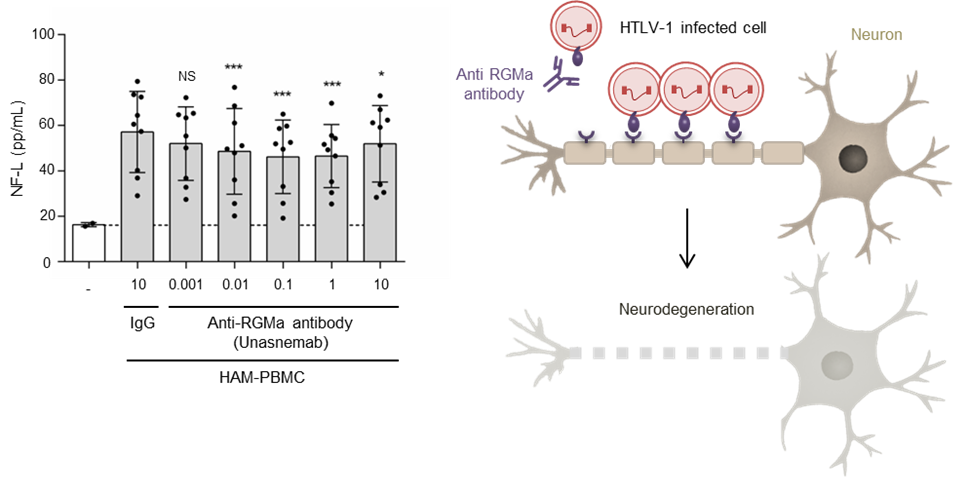
Figure 2. Graph showing reduction of neuronal damage after anti-RGMa antibody treatment (NF-L levels, neurite length)
Publication Information
Title:
Virus-induced RGMa expression drives neurodegeneration in HTLV-1-associated myelopathy
Journal: JCI Insight
Publication date: June 9, 2025,
Authors: Natsumi Araya, Makoto Yamagishi, Makoto Nakashima, Naomi Asahara, Kazuhiro Kiyohara, Satoko Aratani, Naoko Yagishita, Erika Horibe, Izumi Ishizaki, Toshiki Watanabe, Tomoo Sato, Kaoru Uchimaru, Yoshihisa Yamano
DOI: doi: 10.1172/jci.insight.184530.
Related Laboratory
Yamagishi Laboratory, Graduate School of Frontier Sciences, The University of Tokyo


