メディカル情報生命専攻・病態医療科学分野(内丸・中野研究室)がHTLV-1ウイルスタンパク質Rexによる宿主T細胞内でのNMD抑制機構を報告
- ニュース
Elucidation of the Mechanism of Host NMD Suppression by HTLV-1 Rex: Dissection of Rex to Identify the NMD Inhibitory Domain
Kazumi Nakano1*, Nobuaki Karasawa1, Masaaki Hashizume1, Yuetsu Tanaka2, Takeo Ohsugi3, Kaoru Uchimaru1 and Toshiki Watanabe4
- Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Tokyo 108-8639, Japan
- Faculty of Medicine, University of the Ryukyus, 903-0125, Japan
- Department of Laboratory Animal Science, School of Veterinary Medicine, Rakuno Gakuen University, Hokkaido 069-8501, Japan
- Department of Practical Management of Medical Information, Graduate School of Medicine, St. Marianna University, Kanagawa 216-8511, Japan
* Correspondence: nakanokz edu.k.u-tokyo.ac.jp
edu.k.u-tokyo.ac.jp
Viruses 2022, 14(2), 344, DOI: https://doi.org/10.3390/v14020344
<発表のポイント>
Human T-cell leukemia virus typeI (HTLV-1)はヒトT細胞に感染し、時として成人T細胞白血病(ATL)などの重篤かつ予後不良な疾患を引き起こすレトロウイルスである。メディカル情報生命専攻の内丸教授と中野准教授の研究室では、HTLV-1のウイルス機能タンパク質の一つであるRexが、宿主mRNA品質管理機構(nonsense-mediated mRNA decay: NMD)を抑制してウイルスRNAを安定化していることに注目し、そのメカニズムの解明を目指してきた。この度の論文では、本研究室の修士学生の研究成果の積み重ねにより、RexによるNMD抑制機構の分子メカニズムの一端を明らかにした。
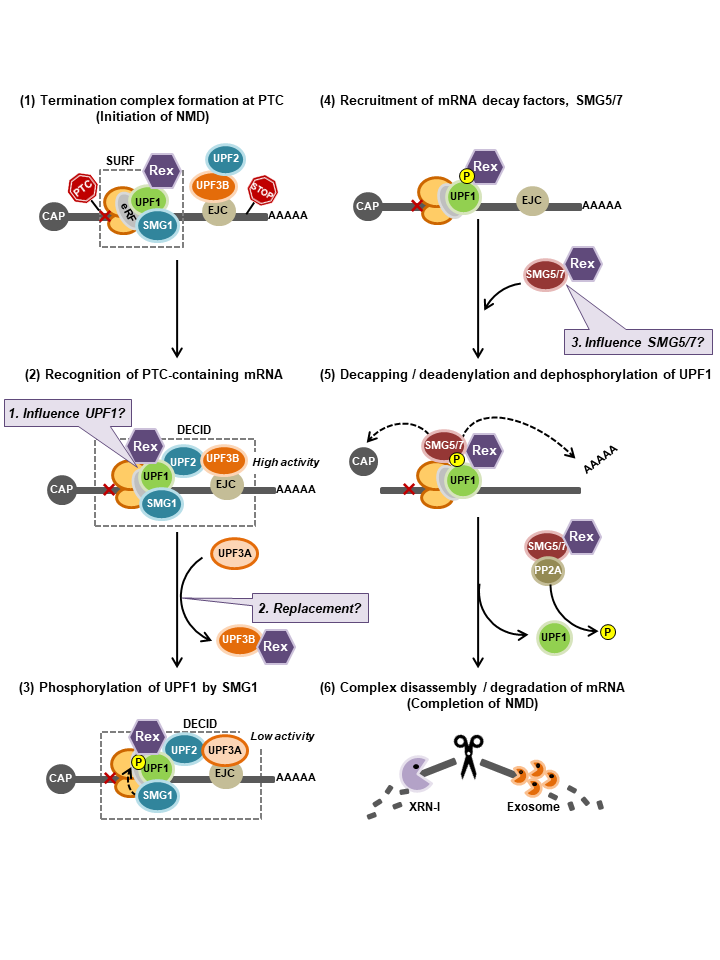
<Graphical Abstract>
Possible model of Rex-NMD complex interaction and new questions. NMD progresses in a cascade as shown in (1-6). (1): Our results suggest that Rex may inhibit NMD by intervening from the beginning to the end of this cascade by interacting with the NMD key regulator Upf1 and may have some effect on its activity (Baloon-1). (2): Rex interacts only with Upf3B, suggesting that Rex substitutes Upf3A for Upf3B in the NMD complex, resulting in the inclusion of less active Upf3A in the NMD complex, thereby suppressing NMD activity (Baloon-2). (4,5): The mRNA decay factors, the SMG5/7 complex, are recruited to phosphorylated Upf1. The SMG5/7 complex recruits the decapping complex (DCP2/DCP1a) and the deadenylation complex CCR4-NOT to enhance decapping and deadenylation of the NMD target mRNA. Additionally, the SMG5/7 complex recruits protein phosphatase 2A (PP2A) for dephosphorylation of Upf1. Since dephosphorylation of Upf1 is essential for NMD completion, Rex may influence the dephosphorylation pathway of Upf1 via SMG5/7 interaction, and thus may influence NMD completion (Baloon-3).